Upcoming Online Panel Discussions
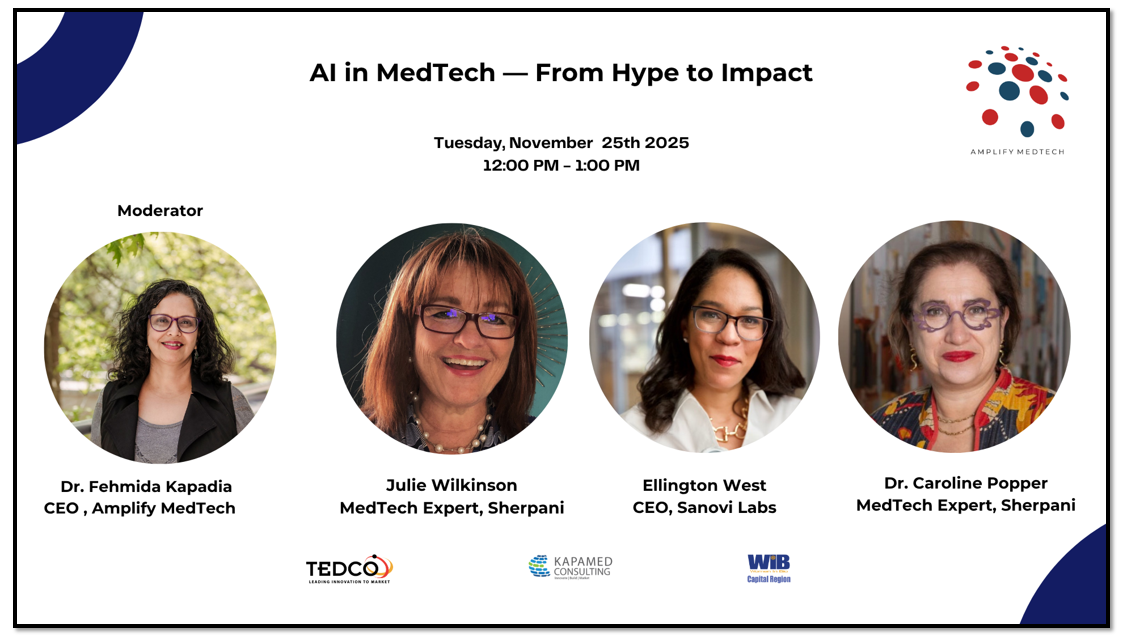
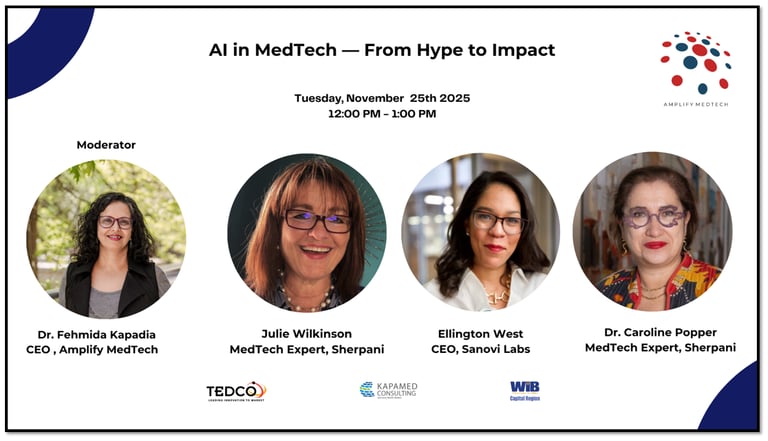
AI in MedTech
Navigating the FDA Maze: Insights from FDA Reviewers and Innovators
Join Julie Wilkinson, Dr. Caroline Popper, and Ellington West for a one-hour online panel exploring how artificial intelligence is moving beyond the buzz to deliver real-world impact in medical technology. Discover how AI is transforming product development, clinical validation, and patient care from smarter diagnostics to equitable access and learn how innovators are bridging the gap between algorithms and outcomes. Gain practical insight into where AI is delivering measurable value today, what challenges remain, and how MedTech companies can integrate AI responsibly and effectively. Learn how investors and healthcare partners evaluate AI-enabled solutions, and what it takes to translate innovation into improved patient outcomes.
Our Latest Discussions
Navigating Clinical Trials: Critical Strategies for MedTech Success.
Non-Dilutive Funding for MedTech Innovators - SBIRs and Beyond.
Regulatory Considerations for AI in MedTech.
Navigating US Healthcare Reimbursement.
IP strategies for Protecting MedTech Innovations in the Digital Age
From Pitch to Investment: MedTech Fundraising Strategies That Work
Product Market Fit: Align Your Product, Strategy, and Commercialization
De-Risking MedTech Product Development: From Idea to FDA
Navigating the FDA Maze
Amplify MedTech’s webinar gave us practical insights on reimbursement and payer engagement—truly impactful for our MedTech journey.
DataPorium

